2007 Schools Wikipedia Selection. Related subjects: Climate and the Weather
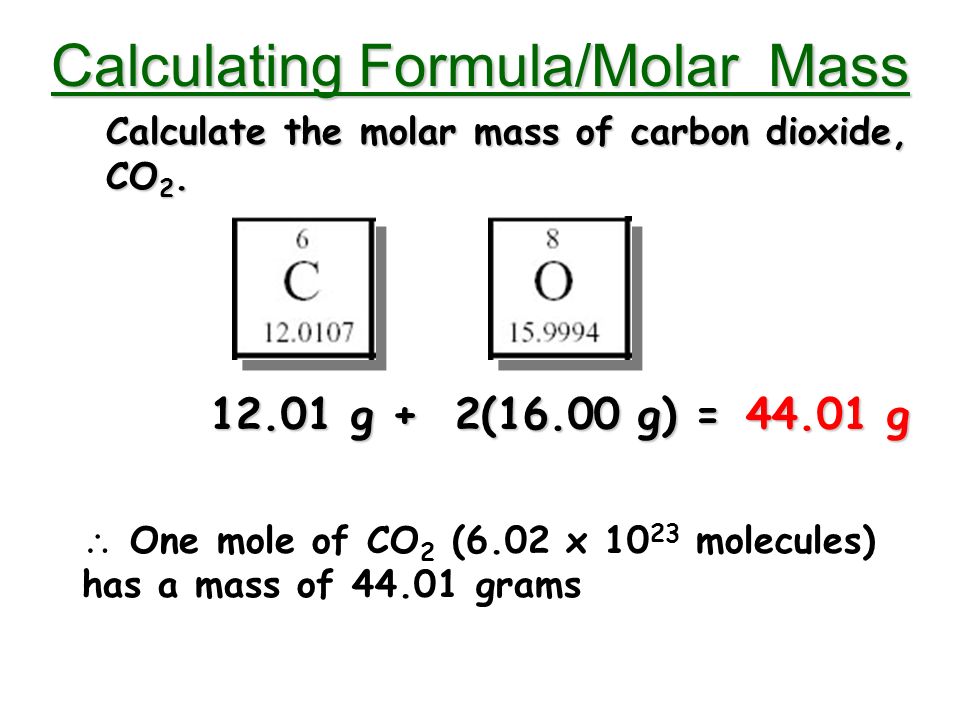
A carbon dioxide molecule has a molecular weight of 44 atomic mass units (the carbon atom contributes 12 AMU, the two oxygen atoms contribute 32 AMU, combined). So, a CO2 molecule is 1.526x. Calculate the maximum mass of carbon dioxide that can be made from 6.0 g of carbon and an excess of oxygen. (Relative atomic masses: C = 12.0, O = 16.0). Calculate the molecular mass of CO2 We need to find the molecular mass of carbon dioxide Molecular mass ofCO 2 = Molecular mass of carbon + 2 (Molecular mass of oxygen) 2 atoms of oxygen are present) Molecular mass of carbon = 12. ›› Carbon Dioxide molecular weight. Molar mass of CO2 = 44.0095 g/mol. Convert grams Carbon Dioxide to moles or moles Carbon Dioxide to grams. Molecular weight calculation: 12.0107 + 15.9994.2 ›› Percent composition by element.
Earth's atmosphere is a layer of gases surrounding the planet Earth and retained by the Earth's gravity. It contains roughly 78% nitrogen, 21% oxygen, 0.97% argon, 0.04% carbon dioxide, and trace amounts of other gases, in addition to water vapor. This mixture of gases is commonly known as air. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation and reducing temperature extremes between day and night.
The atmosphere has no abrupt cut-off. It slowly becomes thinner and fades away into space. There is no definite boundary between the atmosphere and outer space. Three-quarters of the atmosphere's mass is within 11 km of the planetary surface. In the United States, persons who travel above an altitude of 50.0 miles (80.5 km) are designated as astronauts. An altitude of 120 km (75 mi or 400,000 ft) marks the boundary where atmospheric effects become noticeable during re-entry. The Karman line, at 100 km (62 mi), is also frequently used as the boundary between atmosphere and space.
Temperature and the atmospheric layers
The temperature of the Earth's atmosphere varies with altitude; the mathematical relationship between temperature and altitude varies between the different atmospheric layers:
- troposphere: From the Greek word 'tropos' meaning to turn or mix. The troposphere is the lowest layer of the atmosphere starting at the surface going up to between 7 km (4.4 mi) at the poles and 17 km (10.6 mi) at the equator with some variation due to weather factors. The troposphere has a great deal of vertical mixing due to solar heating at the surface. This heating warms air masses, which then rise to release latent heat as sensible heat that further uplifts the air mass. This process continues until all water vapor is removed. In the troposphere, on average, temperature decreases with height due to expansive cooling.
- stratosphere: from that 7–17 km range to about 50 km, temperature increasing with height.
- mesosphere: from about 50 km to the range of 80 km to 85 km, temperature decreasing with height.
- thermosphere: from 80–85 km to 640+ km, temperature increasing with height.
- exosphere: from 500-1000 km up to 10,000 km, free-moving particles that may migrate into and out of the magnetosphere or the solar wind.
The boundaries between these regions are named the tropopause, stratopause, mesopause, thermopause and exobase.
The average temperature of the atmosphere at the surface of earth is 14 °C.
Pressure
Atmospheric pressure is a direct result of the weight of the air. This means that air pressure varies with location and time, because the amount (and weight) of air above the earth varies with location and time. Atmospheric pressure drops by 50% at an altitude of about 5 km (equivalently, about 50% of the total atmospheric mass is within the lowest 5 km). The average atmospheric pressure, at sea level, is about 101.3 kilopascals (about 14.7 pounds per square inch).
Thickness of the atmosphere
Even at heights of 1000 km and above, the atmosphere is still present (as can be seen for example by the effects of atmospheric drag on satellites).
However:
- 57.8% of the atmosphere by mass is below the summit of Mount Everest.
- 72% of the atmosphere by mass is below the common cruising altitude of commercial airliners (about 10000 m or 33000 ft).
- 99.99999% of the atmosphere by mass is below the highest X-15 plane flight on August 22, 1963, which reached an altitude of 354,300 ft or 108 km.
Therefore, most of the atmosphere (99.9999%) by mass is below 100 km, although in the rarefied region above this there are auroras and other atmospheric effects.
Composition
| ppmv: parts per million by volume | |
| Gas | Volume |
|---|---|
| Nitrogen (N2) | 780,840 ppmv (78.084%) |
| Oxygen (O2) | 209,460 ppmv (20.946%) |
| Argon (Ar) | 9,340 ppmv (0.9340%) |
| Carbon dioxide (CO2) | 381 ppmv |
| Neon (Ne) | 18.18 ppmv |
| Helium (He) | 5.24 ppmv |
| Methane (CH4) | 1.745 ppmv |
| Krypton (Kr) | 1.14 ppmv |
| Hydrogen (H2) | 0.55 ppmv |
| Not included in above dry atmosphere: | |
| Water vapor (H2O) | typically 1% to 4%(highly variable) |
Source for figures above: NASA. carbon dioxide (updated 2006). Methane updated (to 1998) by IPCC TAR table 6.1 . The NASA total was 17 ppmv over 100%, and CO2 was increased here by 15 ppmv. To normalize, N2 should be reduced by about 25 ppmv and O2 by about 7 ppmv.
Minor components of air not listed above include:| Gas | Volume |
|---|---|
| nitrous oxide | 0.5 ppmv |
| xenon | 0.09 ppmv |
| ozone | 0.0 to 0.07 ppmv |
| nitrogen dioxide | 0.02 ppmv |
| iodine | 0.01 ppmv |
| carbon monoxide | trace |
| ammonia | trace |
- The mean molar mass of air is 28.97 g/mol.
Heterosphere
Below the turbopause at an altitude of about 100 km, the Earth's atmosphere has a more-or-less uniform composition (apart from water vapor) as described above; this constitutes the homosphere. However, above about 100 km, the Earth's atmosphere begins to have a composition which varies with altitude. This is essentially because, in the absence of mixing, the density of a gas falls off exponentially with increasing altitude, but at a rate which depends on the molar mass. Thus higher mass constituents, such as oxygen and nitrogen, fall off more quickly than lighter constituents such as helium, molecular hydrogen, and atomic hydrogen. Thus there is a layer, called the heterosphere, in which the earth's atmosphere has varying composition. As the altitude increases, the atmosphere is dominated successively by helium, molecular hydrogen, and atomic hydrogen. The precise altitude of the heterosphere and the layers it contains varies significantly with temperature.
Density and mass
The density of air at sea level is about 1.2 kg/m3. Natural variations of the barometric pressure occur at any one altitude as a consequence of weather. This variation is relatively small for inhabited altitudes but much more pronounced in the outer atmosphere and space due to variable solar radiation.
The atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the barometric formula. More sophisticated models are used by meteorologists and space agencies to predict weather and orbital decay of satellites.
The average mass of the atmosphere is about 5,000 trillion metric tons. According to the National Centre for Atmospheric Research, 'The total mean mass of the atmosphere is 5.1480×1018 kg with an annual range due to water vapor of 1.2 or 1.5×1015 kg depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27×1016 kg and the dry air mass as 5.1352 ±0.0003×1018 kg.'
The above composition percentages are done by volume. Assuming that the gases act like ideal gases, we can add the percentages p multiplied by their molar masses m, to get a total t = sum (p·m). Any element's percent by mass is then p·m/t. When we do this to the above percentages, we get that, by mass, the composition of the atmosphere is 75.523% nitrogen, 23.133% oxygen, 1.288% argon, 0.053% carbon dioxide, 0.001267% neon, 0.00029% methane, 0.00033% krypton, 0.000724% helium, and 0.0000038 % hydrogen.
Evolution of the Earth's atmosphere
The history of the Earth's atmosphere prior to one billion years ago is poorly understood, but the following presents a plausible sequence of events. This remains an active area of research.
Atomic Mass Carbon Dioxide Oxygen
The modern atmosphere is sometimes referred to as Earth's 'third atmosphere', in order to distinguish the current chemical composition from two notably different previous compositions. The original atmosphere was primarily helium and hydrogen. Heat from the still-molten crust, and the sun, plus a probably enhanced solar wind, dissipated this atmosphere.
About 4.4 billion years ago, the surface had cooled enough to form a crust, still heavily populated with volcanoes which released steam, carbon dioxide, and ammonia. This led to the early 'second atmosphere', which was primarily carbon dioxide and water vapor, with some nitrogen but virtually no oxygen. This second atmosphere had approximately 100 times as much gas as the current atmosphere, but as it cooled much of the carbon dioxide was dissolved in the seas and precipitated out as carbonates. The later 'second atmosphere' contained nitrogen, carbon dioxide, and very recent simulations run at the University of Waterloo and University of Colorado in 2005 suggest that it may have had up to 40% hydrogen. It is generally believed that the greenhouse effect, caused by high levels of carbon dioxide and methane, kept the Earth from freezing. In fact temperatures were probably very high, over 70 degrees C, until some 2.7 billion years ago.
One of the earliest types of bacteria were the cyanobacteria. Fossil evidence indicates that bacteria shaped like these existed approximately 3.3 billion years ago and were the first oxygen-producing evolving phototropic organisms. They were responsible for the initial conversion of the earth's atmosphere from an anoxic state to an oxic state (that is, from a state without oxygen to a state with oxygen) during the period 2.7 to 2.2 billion years ago. Being the first to carry out oxygenic photosynthesis, they were able to convert carbon dioxide into oxygen, playing a major role in oxygenating the atmosphere.
Atomic Mass Of Carbon Dioxide
Photosynthesizing plants would later evolve and convert more carbon dioxide into oxygen. Over time, excess carbon became locked in fossil fuels, sedimentary rocks (notably limestone), and animal shells. As oxygen was released, it reacted with ammonia to release nitrogen; in addition, bacteria would also convert ammonia into nitrogen. But most of the modern day level of nitrogen are due mostly to sunlight-powered photolysis of ammonia released steadily over the aeons from volcanoes.
As more plants appeared, the levels of oxygen increased significantly, while carbon dioxide levels dropped. At first the oxygen combined with various elements (such as iron), but eventually oxygen accumulated in the atmosphere, resulting in mass extinctions and further evolution. With the appearance of an ozone layer (ozone is an allotrope of oxygen) lifeforms were better protected from ultraviolet radiation. This oxygen-nitrogen atmosphere is the 'third atmosphere'.
This modern atmosphere has a composition which is enforced by oceanic blue-green algae as well as geological processes. O2 does not remain naturally free in an atmosphere, but tends to be consumed (by inorganic chemical reactions, as well as by animals, bacteria, and even land plants at night), while CO2 tends to be produced by respiration and decomposition and oxidation of organic matter. Oxygen would vanish within a few million years due to chemical reactions and CO2 dissolves easily in water and would be gone in millennia if not replaced. Both are maintained by biological productivity and geological forces seemingly working hand-in-hand to maintain reasonably steady levels over millions of years.
